May 2021 | Volume 22 No. 2
Breakthrough in Fight against Liver Disease in Newborns
“What we are doing here is applying science to solve an intractable clinical problem: a clinician-scientist’s quest,” said Professor Paul Tam Kwong-hang in describing his research on liver disease in newborn infants. He went on to quote an editorial in The Lancet from 2017: “Paediatric surgery… is possibly the most challenging subspecialty in which to conduct research, and yet it is pushing the boundaries of biomedical science, seeking innovative, often non-surgical, solutions for intractable problems.”
Professor Tam, who is Li Shu-Pui Professor in Surgery, Director of Dr Li Dak-Sum Research Centre and Chair Professor of Paediatric Surgery in HKUMed’s Department of Surgery, recently led a research team to the discovery that abnormal beta-amyloid deposition is a novel disease mechanism for biliary atresia (BA). The groundbreaking finding, made in collaboration with overseas and Mainland researchers, provides insights into new diagnostic and therapeutic strategies for BA, a devastating liver disease in newborns, the causes of which were hitherto largely unknown.
BA is a life-threatening congenital anomaly proceeding from progressive fibro-inflammatory obstruction of the entire biliary tract in newborn babies, and results in liver failure if untreated, making it possibly the epitome of the aforementioned intractable diseases.
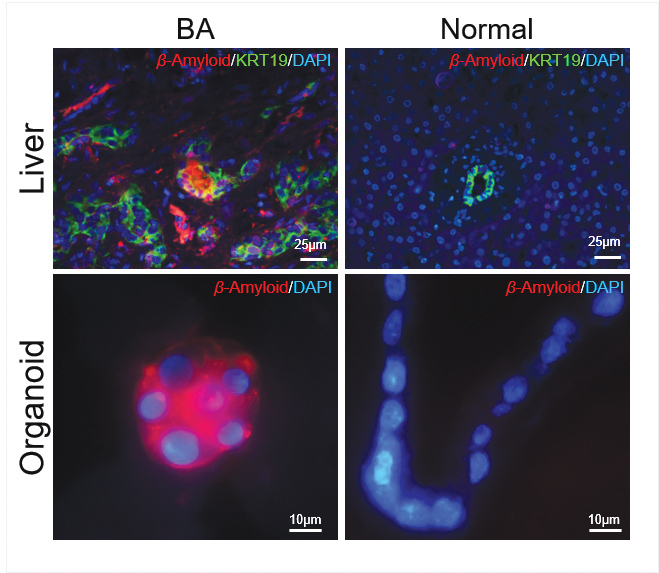
Immunohistochemistry staining for beta-amyloid in human liver tissues, and in human organoids.
Babies suffering
“As a young surgeon, I would see many babies suffering from BA,” said Professor Tam, “and there was a sense of helplessness as the prevailing treatment – surgery – often did not work, resulting in death. And, even when it succeeded in relieving patients’ jaundice, they continue to return to hospital with complications in ensuing years, often for life.”
The aim of the study was to seek a new cure by applying science to discover key factors responsible for the cause and deterioration of the disease. A novel aspect is that the team successfully created a human surrogate disease model – a patient-derived ‘mini-organ’ – that allowed them to discover a key biological factor, beta-amyloid, in disease causation and deterioration.
“Through research on another congenital disease, Hirschsprung’s disease, which has been my lifelong passion,” said Professor Tam, “I came to realise the power of genomics, stem cell and tissue engineering in solving human diseases, and began to feel that a solution for BA could be feasible through the application of precision and regenerative medicine, focussing on human-derived materials for advanced laboratory studies.
“We knew that what had held us back in designing a new solution was that we didn’t understand how the disease occurred and why it deteriorated, and the root cause of this was the lack of a valid disease model to test hypotheses in disease causation and deterioration. Bench and animal findings may not be replicated in clinical setting.”
Organoids
The answer was organoids: an organoid is a cell/tissue-derived culture system that can mimic a real-life (in vivo) organ ‘in a dish’. Intestinal organoids were introduced by a Dutch scientist Hans Clevers in 2009, and subsequently many other types of organoids have been developed, but until now, none for human BA liver. This is the first human BA disease model.
The team successfully grew liver organoids from BA patients and controls, giving them a valid disease model, which in turn gave them new therapeutic targets for pre-clinical testing. They found that the BA organoids showed retarded growth, abnormal morphology and disturbed cell polarity and organisation. Analysis of gene-readouts from the BA organoids revealed a developmental shift from bile duct cells to liver cells and, most significantly, altered beta-amyloid-related gene expression.
“The study provided the first evidence about the key role of beta-amyloid in BA,” said Professor Tam. “This has more far-reaching implications too as beta-amyloid is also a key disease-causing, associated factor for Alzheimer’s disease. Many new drugs are being developed to treat beta-amyloid, so our next step is to test some of these drugs in BA to see if we can improve or cure the disease.”
Describing the next step for his team, Professor Tam said: “BA is likely heterogeneous and multifactorial. Finding a substantial disease factor is exciting, but more research is needed to unravel the full story. We need and are applying for a big grant to reveal the full story and ‘take out’ the disease once and for all.
“We will recruit more patients both in Hong Kong and China, integrate genetic, organoid-derived and clinical data, develop next-generation organoids and test therapeutic targets to inform us in designing new clinical trials as final proof of effective new treatments which are likely to be personalised for different patient subgroups and individuals.”
The study provided the first evidence about the key role of beta-amyloid in biliary atresia (BA)… Many new drugs are being developed to treat beta-amyloid, so our next step is to test some of these drugs in BA to see if we can improve or cure the disease.

PROFESSOR PAUL TAM KWONG-HANG

