November 2021 | Volume 23 No. 1
Cover Story
The ‘One Health’ Challenge
What does One Health mean in practice? Professor Malik Peiris, Tam Wah-Ching Professor in Medical Science, of the School of Public Health, one of the most highly cited scholars in the world on emerging infectious diseases and recent joint recipient of the prestigious 2021 John Dirks Canada Gairdner Global Health Award with colleague Professor Guan Yi, Daniel C K Yu Professor in Virology, provides a telling example based on his work as a virologist in Sri Lanka in the 1980s.
He specialised back then in mosquito-borne diseases, and Japanese encephalitis was on the rise in a region of the country. After much investigation, the cause was found to be a well-intentioned policy: the government decided to help poverty-stricken rice farmers by giving them pigs to raise. The combination of mosquitoes and pigs – which are often a vector of viruses between humans and other animals – “just lit the spark to the dynamite,” he said.
“I’m sure the health department wouldn’t have thought of this happening either. It just shows how these ecological balances can be upset by some of the most well-meaning gestures.”
Such interplay between humans, animals and the environment is at the heart of the One Health concept, which started to consolidate after outbreaks of H5N1 bird flu and SARS (both centred in Hong Kong) and was formally endorsed by the World Health Organization (WHO), Food and Agriculture Organization and World Organisation for Animal Health in 2010. It has leapt up the agenda in the wake of COVID-19, which has been the latest and worst of recent viruses that threaten human health.
“For the last two decades, we have had a major, novel infectious disease threat almost every two or three years – SARS, swine flu, avian flus H5N1 and H7N9, MERS, Zika, Ebola and now COVID-19,” Professor Peiris said. All have been traced to animal sources – birds, bats, pigs, camels and primates – but human interactions with these animals have created conditions that allow viruses to jump species.
“Previously, livestock was raised in a backyard. People had a few pigs or chickens and if a virus got into those animals and jumped to humans, it was a localised effect,” he said. “Now, livestock animals are raised in the tens of thousands and shipped thousands of miles to market. You also have these game animal markets, which have become a huge trade because of greater affluence in China and parts of Asia, and you can have thousands of animals in the larger markets. That is, of course, how SARS managed to develop.
“COVID-19 really should be a wake-up call to the global community that humans are not superhuman. The forces of nature are much more powerful than us and we tamper with this at our peril.”

Professor Malik Peiris (second from right) and his team found that novel coronavirus can infect the human respiratory tract even better than SARS-CoV.

An electron microscope picture shows that the cells from human bronchial tissue can be infected by MERS-CoV and the MERS-CoV can replicate in human respiratory tissues.
COVID-19 really should be a wake-up call to the global community that humans are not superhuman. The forces of nature are much more powerful than us and we tamper with this at our peril.

PROFESSOR MALIK PEIRIS
Viruses not the only threats
Professor Peiris and other scholars at HKU are at the frontlines trying to assess the threats emerging from the human-animal-environment interface and propose ways to manage them. The concern is not confined to viruses but also antimicrobial resistance (AMR), which is developing more slowly than new viruses but has the potential to be very damaging to health.
AMR was identified by the United Nations Environment Programme (UNEP) in 2017 as one of the top six emerging issues of global environmental concern. Currently, about 700,000 people die each year because AMR has made antibiotics less effective against bacterial infections. By 2050, the number could be 10 million (twice as many as died of COVID-19 in its first 18 months) if nothing is done, according to a UK-commissioned report. A 2019 report by the UN’s Interagency Coordination Group on Antimicrobial Resistance, titled ‘No Time to Wait’, also warned of the growing impact of AMR on the environment and ecological systems.
Professor Zhang Tong of the Department of Civil Engineering leads an ongoing theme-based research project to investigate AMR flows from pollution hotspots to the environment, with a focus on sewage. He works in collaboration with HKU’s School of Public Health and the Hong Kong Government and is also a member of an expert panel preparing a special report on the environmental impact of AMR for a special session next year of the UNEP.
“It is impossible to completely remove antibiotics from wastewater so some inevitably escapes into the environment,” he said – whether from human or animal farm sewage or the effluents of the pharmaceutical industry. The residual antibiotics may apply selective pressure on bacteria, some of which will develop resistance to these antibiotics. “Eventually, there will be more and more superbugs.”
He is in the process of collecting water and soil samples from multiple sites, such as hospitals, water treatment plants, farms, beaches and sewage treatment plants, with the aim of developing a local map of the current baseline conditions that can be used to monitor AMR progress in Hong Kong’s environment and develop control strategies. The baseline will also be compared to other territories to see how Hong Kong fares globally.
This detection work is made possible by DNA technology Professor Zhang developed to detect antibiotic-resistant genes in water samples, which has been widely adopted by researchers across the world. He has also applied molecular detection work to identify the SARS-CoV-2 virus that causes COVID-19 in local sewage samples.
Using an RNA-based technology he developed and working with HKU’s School of Public Health, Professor Zhang and his team began to detect the virus last November, days before cases were confirmed. This led to the government introducing compulsory testing for places with positive signals from sewage samples. The team have since developed a method to identify variants of the virus.
“COVID-19 is an example of how we understand One Health from another angle. We see the flow of pathogen from human to environment. We can use information from one compartment to indicate the situation in another. The same thing applies to antibiotic-resistant genes. We survey the environment to not only look at the possible sources of AMR in humans and animals, but also as a reflection of what may be happening in humans and animals,” he said.

Extraction of SARS-CoV-2 RNA from sewage using an automatic extraction machine.

Professor Zhang Tong (right) giving a presentation to the HKSAR Chief Executive Mrs Carrie Lam, HKU Council Chairman Professor Arthur Li and HKU President Professor Xiang Zhang on the work of the Environmental Microbiome Engineering and Biotechnology Laboratory.
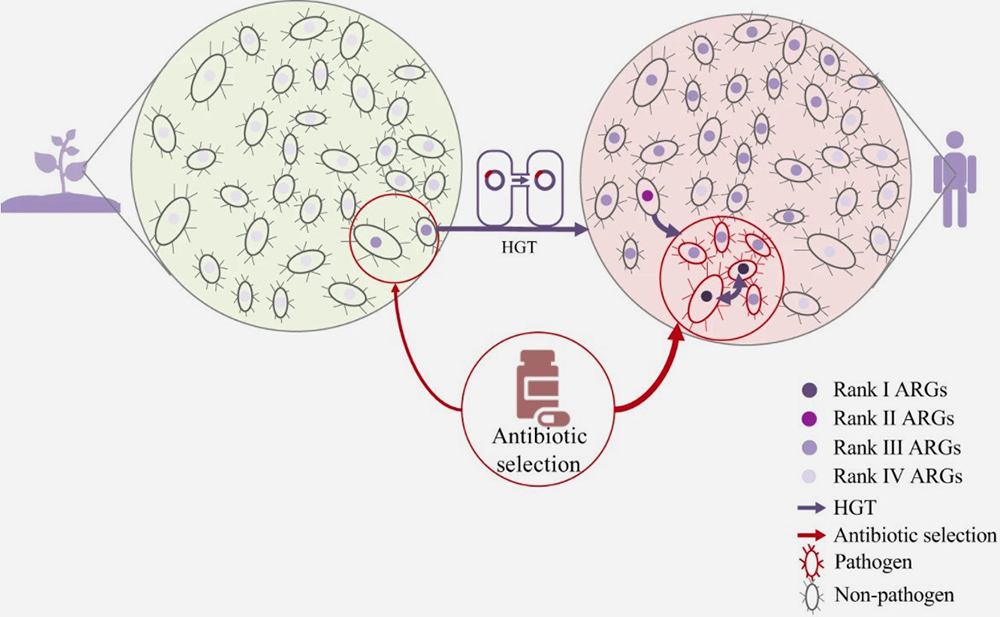
A conceptual model to demonstrate the evolution and emergence of antibiotic-resistant genes accelerated by selective pressure of antibiotics.
It is impossible to completely remove antibiotics from wastewater so some inevitably escapes into the environment… Eventually, there will be more and more superbugs.

PROFESSOR ZHANG TONG
Our gut contributions
One of Professor Zhang’s collaborators has been Dr Hein Tun, a veterinary public health specialist in the School of Public Health who has multiple projects of his own on the nonhuman sources of human AMR.
For instance, an ongoing study is measuring AMR levels and transmission across beaches, healthy people, hospital patients, farms, rivers and creeks in Hong Kong, Mainland China and Thailand. So far, the data shows AMR levels are higher in animal production than in people. Dr Tun is also in the process of studying the levels and sources of AMR in hospital wastewater, which does not require special treatment in Hong Kong before being discharged into the sewage system, and how AMR may be transmitted in the household, such as via countertops or other surfaces.
“The One Health concept is very useful not only for COVID-19 but other types of diseases. Whether you look at the community level, the global level or the individual level, this concept can apply. You cannot stay away from the environment,” he said.
Dr Tun’s work on a particular type of AMR bacteria, ESBL-E, has been revealing of how environmental and human factors interact. The WHO has prioritised ESBL-E because it can transfer AMR to other bacteria, so if someone with ESBL-E in their gut is exposed to salmonella, it can transfer resistance to the salmonella, making it very difficult to treat it with antibiotics. In a study of 90 Hong Kong travellers that measured their gut microbiota before and after travelling, he found exposure to raw seafood was a factor in those who acquired ESBL-E after their trips (although about 40 per cent tested positive before travelling). People were also more likely to have ESBL-E if their gut lacked bifidobacteria, which are beneficial.
“This is why carriage of AMR bacteria is so important. If healthy people carry it in their gut, those bacteria can share the resistant gene to pathogenic bacteria and put their health at risk,” he said.
Dr Tun also studied AMR in the wake of COVID-19 among some members of the traveller cohort. There was speculation that AMR would increase as people self-medicated with antibiotics, which the study seemed to support – participants had more AMR bacteria as well as chemicals related to masks in their guts. “We are trying to recall these participants to study the health impacts of this,” he said.
“We need to think collectively, across different sectors and expertise, to have more of the multidisciplinary and interdisciplinary collaboration that we’ve seen with COVID-19. It’s also important to have participation from the public,” he said. Dr Tun has been promoting citizen science in less-developed countries to involve residents in collecting data, such as exposure to animals and the environment, through their phones. “This way they can know what their risk is and what the problems are. They can have a sense of ownership of the data and findings.”
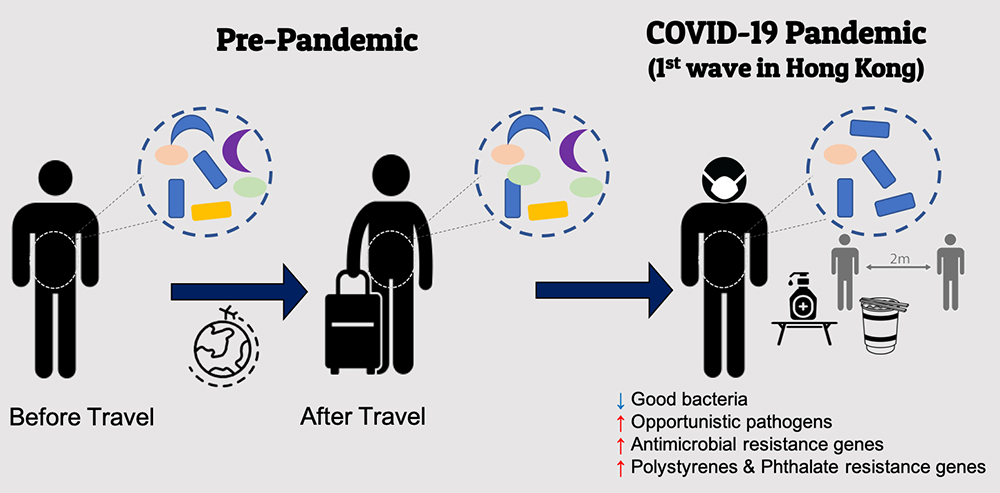
Increased opportunistic pathogens and resistance genes in the gut microbiome during the first wave of COVID-19 in Hong Kong.
The One Health concept is very useful not only for COVID-19 but other types of diseases. Whether you look at the community level, the global level or the individual level, this concept can apply. You cannot stay away from the environment.

DR HEIN TUN
Stark warning
That need for collective purpose is echoed by Professor Zhang and Professor Peiris. The concept of One Health should be an easy sell, Professor Zhang said. “Especially in China because Chinese philosophy tells us that everything is connected, that it’s not separate.” He sees the environmental sector as best placed to lead the way, such as through UNEP.
Professor Peiris, however, has seen firsthand the difficulties of having policymakers and businesses monitor and respond to health threats – in the lead-up to the H7N9 outbreak in China, the poultry trade had little incentive to monitor for viruses because any infections meant they would have had to cull their stock. Similarly with MERS, the camel industry in Saudi Arabia has been resistant to evidence that the MERS virus comes from camels.
MERS remains a pandemic threat. Recent research by Professor Peiris and international collaborators showed that it is present in camels across North Africa and the Middle East. It is still in a milder form than the Saudi Arabian version, but the worry is that it, too, could evolve to be more pathogenic in humans. “We have to be concerned because MERS is repeatedly jumping to humans. There is no reason why it cannot further adapt to become more efficiently transmissible to humans,” he said.
Professor Peiris sees infectious disease threats in the wider context of the great challenges facing the planet and the similar urgent need to collaborate and break down institutional and other barriers in order to find solutions. “It’s not just infectious disease. You can see issues of climate change, environmental pollution, loss of biodiversity – we are really rupturing the limits of sustainability of our planet. I think it’s important for everybody’s education, but particularly for medical education, for us to realise that it’s not just about treating individual humans. We have to take account of animals and the environment, too. We have to be advocates.
“If we want human health to be better, we have to look after planetary health, environmental health and animal health,” he said, adding this cautionary note: “Rene Dubos, a French microbiologist, said in 1959 that ‘at some unpredictable time and in some unforeseeable manner, nature will strike back’. COVID-19 is a great illustration of that.”

Professor Zhang Tong (centre) with Laboratory Manager Ms Vicky Fung (left) and Research Assistant Professor Dr Yu Deng (right).

